5 Regulatory Affairs Bottlenecks Pharma Companies Could Solve with Agentic AI
Struggling with regulatory affairs bottlenecks? Explore how pharma leaders can leverage Agentic AI to transform regulatory workflows and accelerate approvals.
Aishwarya
10/26/20256 min read


Introduction
Regulatory Affairs teams in the pharmaceutical industry are drowning in documentation, constantly changing global requirements, and time-critical submissions. Every delay in submitting a New Drug Application (NDA), Marketing Authorisation Application (MAA), or Investigational New Drug (IND) carries significant consequences.
Recent research from Tufts Center for the Study of Drug Development reveals that each day of delay costs approximately $500,000 in lost prescription drug or biologic sales. At the same time, direct clinical trial expenses add another $40,000 per day. For patients, these delays mean prolonged suffering and delayed access to potentially life-saving therapies.
The regulatory landscape is increasingly complex across key markets:
United States: U.S. Food and Drug Administration (FDA)
United Kingdom: Medicines and Healthcare products Regulatory Agency (MHRA)
European Union: European Medicines Agency (EMA)
Global harmonization: International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH)
Phase III clinical trials now cost an average of $36.58 million, representing a 30% increase from 2018 levels, driven by growing protocol complexity and operational challenges. Traditional automation tools such as Robotic Process Automation (RPA) can handle repetitive tasks, but they cannot reason, adapt, or act autonomously when faced with regulatory complexity.
This is where Agentic Artificial Intelligence (Agentic AI) delivers a step-change. In this blog, we deep dive into the top 5 regulatory affairs bottlenecks pharma leaders can solve with Agentic AI.
What Makes Agentic AI Different?
Agentic AI is a system that can understand context, make decisions, and execute multi-step tasks autonomously; not just generate text or respond to prompts.
Unlike predictive AI, which only responds when prompted, Agentic AI can:
Monitor rules, data, or systems in real time
Take action across workflows without human intervention
Collaborate with humans through human-in-the-loop oversight
Learn and improve processes through continuous feedback
For Regulatory Affairs, this means fewer manual workloads, dramatically reduced errors, and faster submissions. According to Gartner, by 2028, one-third of interactions with generative AI services will use autonomous agents for task completion, fundamentally transforming how regulatory teams operate.
Think Agentic AI is too complex to apply? Industry leaders with insights from our eBook “Agentic AI for Business Leaders” are simplifying workflows and gaining back 30+ hours each month. Grab your FREE copy now!
The 5 Biggest Regulatory Affairs Bottlenecks Agentic AI Can Solve
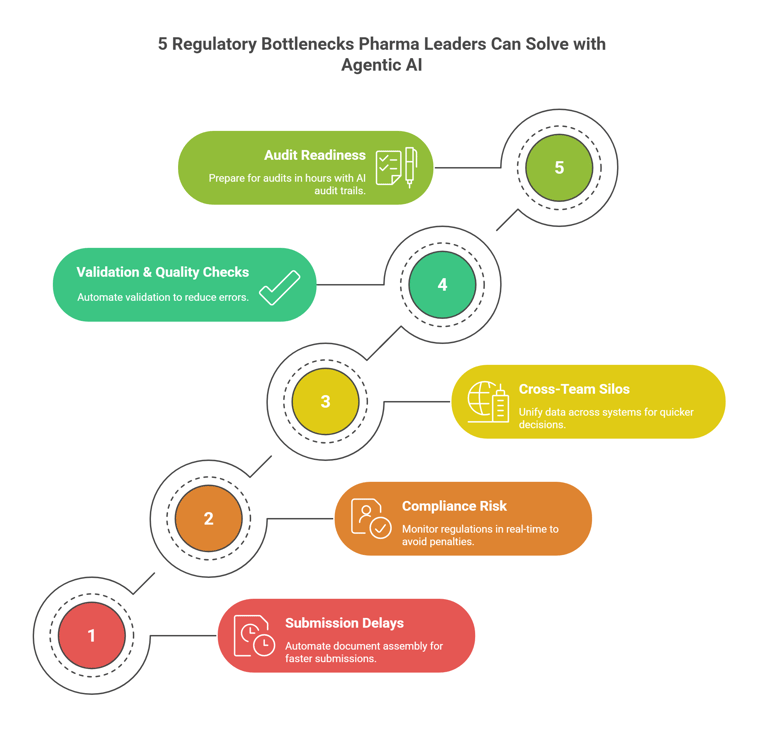
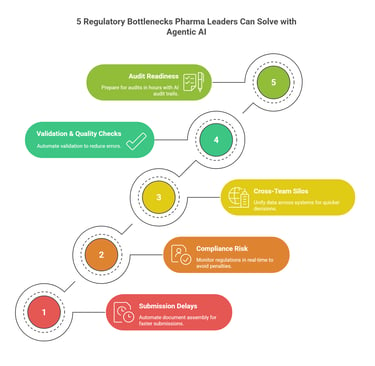
1. Submission Delays from Documentation Overload
The Problem:
Regulatory submissions span thousands of pages, including investigator brochures, clinical summaries, quality modules, labeling, variations, updates, and more. Manually preparing and validating eCTD (electronic Common Technical Document) modules creates significant delays and increases the risk of formatting errors that trigger costly resubmissions.
How Agentic AI Solves It:
Agentic AI can:
Auto-assemble submission-ready documents from disparate data sources
Validate structure, cross-references, and regulatory template compliance
Maintain version control with full audit traceability
Identify missing sections before submission
Business Impact:
40-60% faster submission preparation time
Minimize costly rework cycles from formatting errors
Accelerate time-to-market by 3-6 months per product
Free regulatory writers to focus on strategic content
2. Compliance Risk from Constantly Changing Regulations
The Problem:
FDA, EMA, MHRA, ICH, and regional agencies frequently update compliance rules, guidance documents, and submission formats. Missing a regulatory change can trigger audits, submission delays, or outright rejection, yet tracking changes across multiple jurisdictions manually is nearly impossible.
How Agentic AI Solves It:
Agentic AI can:
Monitor regulatory authority updates in real time across global markets
Map regulatory changes to impacted products or documents
Recommend next steps and automate stakeholder notifications
Draft compliance plans and update internal SOPs
Business Impact:
Real-time regulatory intelligence across 50+ markets
Lower compliance risk and avoid regulatory penalties
Proactive rather than reactive compliance management
Inspection readiness maintained continuously
3. Cross-Team Silos Slowing Regulatory Intelligence
The Problem:
Regulatory data is scattered across Clinical Trial Management Systems (CTMS), Quality Management Systems (QMS), Pharmacovigilance (PV) databases, Manufacturing systems, and Document Management Systems (DMS). Deloitte research identifies that pharmaceutical companies' rising R&D costs can be attributed to several factors, including continuing to operate in functional silos. Research shows that 48% of senior decision-makers in drug development companies said data silos derailed the efficiency of cross-functional collaboration.
Teams waste hours searching for information buried in disconnected systems, delaying critical decisions.
How Agentic AI Solves It:
Agentic AI can:
Connect siloed systems and unify regulatory insights
Answer natural-language queries (e.g., "Show EU submission status for Product A")
Push real-time updates to relevant stakeholders automatically
Create unified dashboards with cross-functional visibility
Business Impact:
50-70% reduction in time spent searching for information
Faster decision-making across regulatory, clinical, and quality teams
Eliminate email-based bottlenecks and manual status updates
Single source of truth for all regulatory intelligence
4. Time-Consuming Validation & Quality Checks
The Problem:
Formatting validation, proofreading, cross-referencing citations, and template compliance checks are repetitive, error-prone tasks that consume significant regulatory team time. Yet these tasks carry high compliance impact. Mistakes can result in audit findings or submission rejection.
How Agentic AI Solves It:
Agentic AI can:
Enforce formatting rules and regulatory templates automatically
Validate data fields, citations, and cross-references
Flag missing, inconsistent, or non-compliant sections
Perform quality checks at scale across thousands of pages
Business Impact:
60-75% reduction in validation cycle time
Fewer audit findings and compliance issues
Higher submission quality with minimal human review
Regulatory writers focus on content, not formatting
5. Slow Audit & Inspection Readiness
The Problem:
When regulatory auditors or inspectors request documents, decision logs, correspondence trails, or compliance evidence, teams scramble through folders, email inboxes, and multiple systems. This last-minute preparation is stressful, time-consuming, and increases the risk of missing critical documentation.
How Agentic AI Solves It:
Agentic AI can:
Maintain AI-driven audit trails automatically
Retrieve requested evidence instantly with intelligent search
Summarize findings and generate audit-ready reports
Track all regulatory interactions and decisions continuously
Business Impact:
Inspection readiness in hours, not weeks
Reduced audit preparation stress and overtime
Complete documentation trails with zero gaps
Stronger regulatory relationship through rapid response
Why the Shift Is Urgent for Pharma Leaders
Gartner predicts that by 2028, around 33% of enterprise software applications will include Agentic AI, up from less than 1% in 2024. The pharmaceutical industry cannot afford to lag behind this transformation.
Deloitte's research suggests that pharmaceutical organizations have an opportunity to unlock $5 to $7 billion in value, with R&D representing the top value opportunity at 30 to 40%. Companies that adopt Agentic AI early will gain compounding advantages: faster approvals, lower costs, reduced compliance risk, and the ability to bring life-saving therapies to patients sooner.
Regulatory complexity will only increase with accelerated approval pathways, real-world evidence requirements, digital therapeutics regulations, and post-pandemic policy shifts. Early adopters will establish market leadership while competitors struggle with manual processes.
Getting Started with Agentic AI: A Practical Roadmap
Implementing Agentic AI in regulatory affairs doesn't require a complete organizational overhaul. Here's how pharma leaders can begin:
Phase 1: Identify Your Biggest Bottleneck (Weeks 1-4) Start with one high-pain area: document compilation, adverse event processing, or compliance tracking. Quick wins build organizational confidence and create ROI justification for broader rollout.
Phase 2: Pilot with Existing Data (Days 30-90) Agentic AI learns from your historical data: past submissions, regulatory correspondence, SOPs, and audit findings. A focused 90-day pilot can demonstrate tangible value with minimal disruption to current operations.
Phase 3: Scale Strategically (Months 4-9) Once proven, expand AI capabilities across other regulatory functions. Integration with existing systems (document management, clinical trial databases, safety systems) maximizes value and minimizes change management friction.
Phase 4: Continuous Optimization (Ongoing) Agentic AI improves over time through learning. Regular model retraining based on new regulations, regulatory feedback, and submission outcomes ensures sustained performance gains and keeps pace with regulatory evolution.
Explore our blog section for in-depth insights and real-world strategies on how leaders across industries are adopting Agentic AI to build smarter, more efficient operations.
How Elevin Helps Regulatory Teams Automate Safely
At Elevin Consulting, we understand that regulatory affairs requires precision, auditability, and unwavering compliance. That's why our Agentic AI solutions are built with regulatory validation frameworks from day one.
With Elevin's Agentic AI Consultation, pharma organizations get:
Custom AI agents for submissions, compliance monitoring, and audit workflows
Secure integration with existing RA, Quality, and Clinical systems
Human-in-the-loop oversight and validation protocols
Compliance frameworks aligned with FDA, EMA, MHRA, and ICH standards
90-day proof-of-value implementations with measurable results
We partner with pharmaceutical and biotech companies to identify the highest-impact opportunities, design practical implementation roadmaps, and deliver measurable improvements, typically within the first quarter.
Take the Next Step
If regulatory workloads are slowing your approvals, delaying time-to-market, or creating compliance risks, it may be the right time to explore how Agentic AI can transform your regulatory operations.
Book a free 30-minute discovery call to discuss your specific regulatory challenges and discover how Elevin's Agentic AI solutions can accelerate submissions, reduce compliance risk, and shorten approval timelines for your organization.
Excellence
Elevin Consulting: Your Partner in Growth.
Impact
© 2025 Elevin Consulting Pvt Ltd. All Rights Reserved
Trust
hello@elevinconsulting.com
